Efficacious Molecular Modelling
Advanced Molecular Modeling and Simulation

When generating graph-based molecular compounds, a common challenge is ensuring that the generated structures comply with essential biochemical and physical constraints. Failure to meet these constraints can render proposed molecules invalid for practical use in drug discovery or chemical engineering.
Intractābilis has developed an advanced diffusion model based molecular modelling paradigm that addresses this issue through the preservation of network motifs. The approach involves compressing key structural elements, such as subsets of rings to retain critical molecular substructures while allowing for more efficient and targeted manipulation during the generative process. The number of nodes and edges eligible for state changes at each diffusion step is directly linked to the time-step, allowing the model to progress from smaller subgroups to increasingly complex structures in a controlled manner. This incremental strategy accelerates the learning process and reduces the computational time required for molecule generation.
Our solution significantly simplifies decoding, leading to faster and more reliable generation of chemically valid molecules. The result is a powerful and efficient framework for molecular innovation that retains structural fidelity while enabling scalable exploration of chemical space.
Geometric Representation Learning for Molecular Interaction Modelling
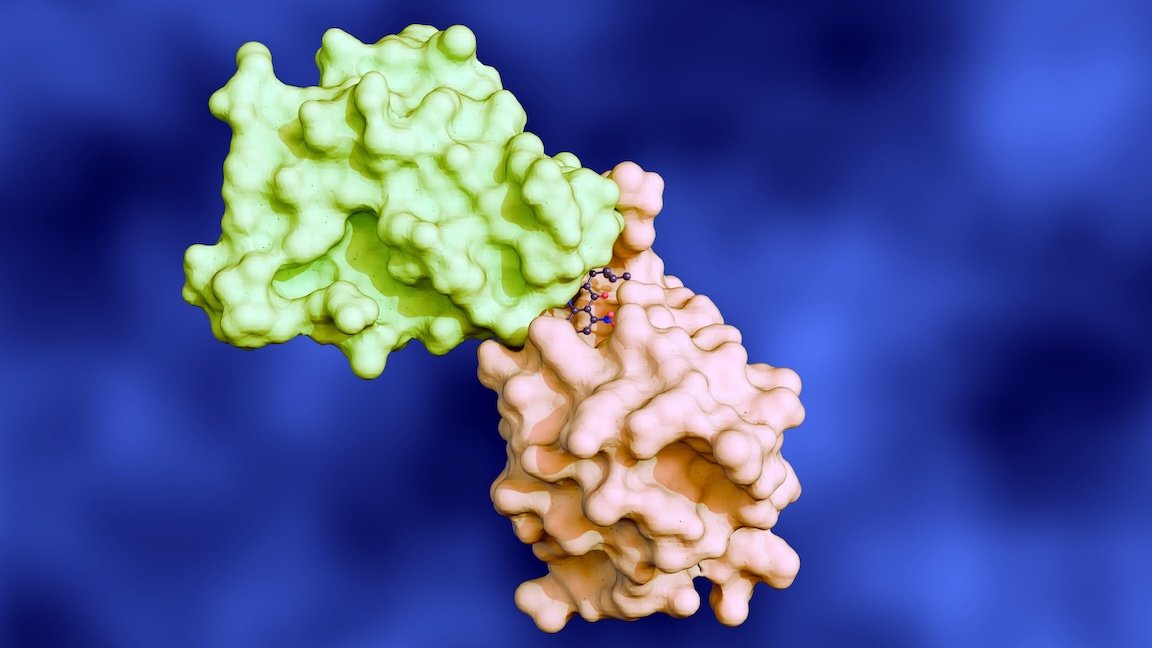
The expansive time and financial costs of conducting wet-lab experiments to test the interaction behaviour of all possible molecular pairs present a significant barrier to scientific progress. Such experiments are resource-intensive, particularly given the vast number of potential molecular combinations.
As molecular structures are chaotically distributed in space, their interactions are inherently geometric in nature. It is therefore necessary to account not only for the geometry of each individual molecule but also for their relative positions and orientations. Acquiring this level of detail is a daunting task, making it challenging to fully capture the geometry of interaction environments. In addition to spatial complexity, the forces at play during molecular interactions are critical to understanding a wide range of physical, chemical, and biological processes. Accurate modelling of these forces provides insights into behaviours that are otherwise difficult or costly to measure experimentally.
Intractābilis has developed a novel molecular representation learning protocol that models interaction environments using geometric information. Our solution enables the prediction of molecular interaction dynamics across diverse contexts, from physical property estimation to biological function analysis.
Foundation Model for Natural Cosmetic Compounds Discovery

The development of cosmetics and skin-care products, particularly those inspired by natural sources, has historically relied on empirical, and trial-and-error methods. While natural products dominate cosmetic innovation and are a preferred choice among consumers, identifying compounds that can be directly translated into market-ready formulations is both time-intensive and costly. The structural diversity of natural products is vast, encompassing hundreds of thousands of unique molecular architectures. These include unconventional scaffolds, complex stereochemistry, polycyclic ring systems, and functional groups rarely found in synthetic chemical libraries, which often invalidates standard cheminformatics assumptions.
Intractābilis has addressed these challenges by developing a specialised foundation model for natural cosmetic products with an innovative pre-training framework. This model integrates masked learning with contrastive learning, enabling it to support a wide range of downstream tasks in natural product research. By leveraging extensive libraries of unlabelled molecular structures and applying biologically inspired pretraining strategies, the model captures the intricate interrelationships between molecular scaffolds with exceptional fidelity.
The foundation model can be adopted for streamlining the research workflows for beauty product innovation and accelerating discovery cycles. By combining structural insight with scalable machine learning, it offers both a scientific and commercial advantage.
